Written by: Bita Nouri / Kohan Textile Journal
Surfactants are organic compounds, which once added to water or any other liquid, decrease its surface tension, thereby causing the liquid to effortlessly spread over the surface of a material or increase the ability of a liquid to penetrate into the material.
In other words, they lower the surface tension between two liquids or a liquid and a solid.
Textile surfactants play an important role in the textile industry as one of the compounds used as antistatic, wetting, untangling, and softening agents in different processes of textile manufacturing such as scouring, dyeing and finishing.
Disposal of synthetic surfactants into the marine ecosystem cause, environmental problems that limit the growth of the synthetic textile surfactants market. Thus the demand for bio-based textile surfactants has dramatically raised. This article provides a general overview of kelp that is a kind of brown algae, its features, and uses of the kelp surfactant as a new class of biodegradable and biocompatible surfactant.
1- Introduction
Chemical surfactants are widely used in various industries. However, one of the environmental concerns is the effluent of chemical surfactant that has a detrimental effect on the aquatic and marine ecosystem. Consequently, natural surfactants such as kelp surfactants have gained increasing attention due to their unique properties [1].
2- Kelp
The giant kelp, Macrocystis Pyrifera, is a kind of brown algae seaweeds grows predominantly in South Africa (the Atlantic and the Indian Ocean), South America, South Australia, and the western Pacific Ocean.
Kelps are known as the fastest-growing organisms on the earth as their high growth rate can reach an average height of 30 to 80 meters.
Kelps grow in cold and shallow brackish water and live up to seven years. Areas, where these algae grow densely, are called kelp forests.
Algae are the only seaweeds that have air bladders. Without air bladders, they would be too heavy to float on the surface of the sea and since they produce their own food from the sunlight, they wouldn’t be able to photosynthesize. Figure 1 shows kelp forests and kelp leaves with its air bladders [2, 3].

3- Application of kelp
Seaweeds are important marine Bio-based materials. Kelp has been used in different fields, especially in cosmetics, pharmaceuticals, biofuels, and wastewater treatment. Here are some uses of kelps [2, 6, 5].
3-1- Food recourse
It has been a long time that kelps have been used and also domestically farmed as a food resource. It is rich in iodine, potassium, as well as a large variety of other minerals. It is used in cooking in many of the ways that other vegetables are used [6, 2].
3-2- Soda ash
Historically the supply of soda ash, made mostly from burning plants and seaweeds such as kelps. Sodium carbonate has many applications and is an important ingredient which is best known for being used in detergents and cleaning products [6].
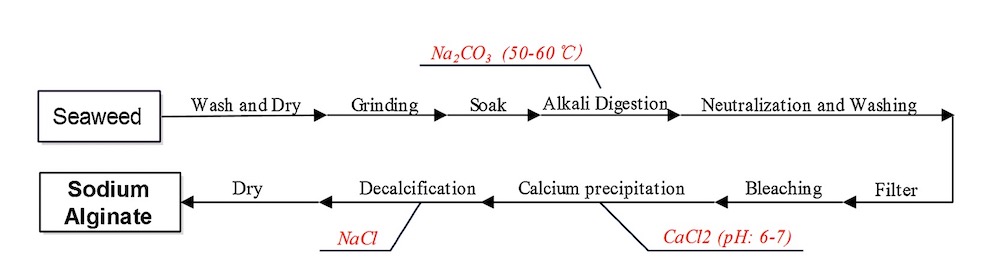
3-3- Alginate
Alginate is a natural linear anionic polysaccharide typically obtained from the cell wall of algae, including kelp, and composed of α-d-mannuronic acid and β-l-guluronic acid that is derived from seaweed [7].
It is widely used in various industries as a thickener, emulsifier, and stabilizer. In addition to this, the demand for alginate has increased over time due to its biocompatibility, low toxicity and gelling properties by the addition of divalent cations such as Ca2+ [8].
Alginate hydrogel has widely used in biomedical and bioengineering as a natural, biological hydrogel. It is a good candidate for burns and wound management. Also, the gel-forming property of alginate helps in removing the wound dressing without pain. Especially Calcium alginates are indicated for bleeding wounds [7]. In figure 2 and 3 the production of alginate from seaweeds and the formation of an alginate gel by calcium cations are shown.
Marine algae represent a rich source of complex polysaccharides and oligosaccharides that are used as components for green surfactants. Synthesize of surfactants can be operated in two general ways. 1. Directly from kelp 2. From extracted alginate [9, 10].
3-4-1 Synthesize of surfactant from alginate
The surfactant is derived from mannuronic acid, which first must transform into monomeric form. Accordingly, glycosidic bonds must break. This is possible by acid hydrolysis and depolymerizing enzymes. However, due to the strength of this bond, the monomeric form cannot completely obtain, but Partial depolymerization by acid is easily achievable [9].
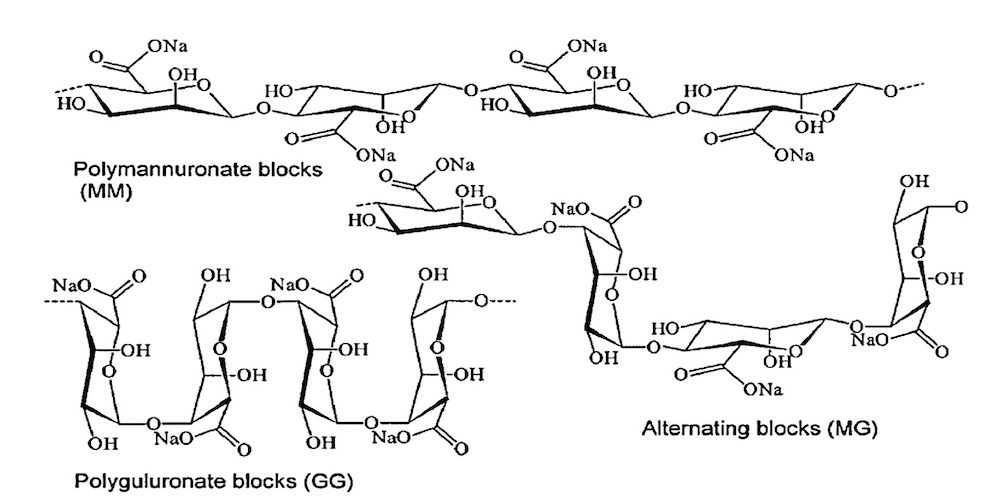
Several researchers have studied the preparation of oligomannuronates by acid and/or enzymatic depolymerizations [15]. Haug and co-workers defined the partial acid depolymerization of alginate to provide homopolymer blocks of mannuronic acid (MM) and guluronic acid (GG) with a degree of polymerization (DP) of 20.
In Fig4 Chemical structures of G-block, M-block, and alternating block can be seen.
Shimokawa [11] and Matsubara [12] reported the preparation of DP1–DP9 or DP1–DP12 saturated oligoguluronate mixtures in 11–12% yields.
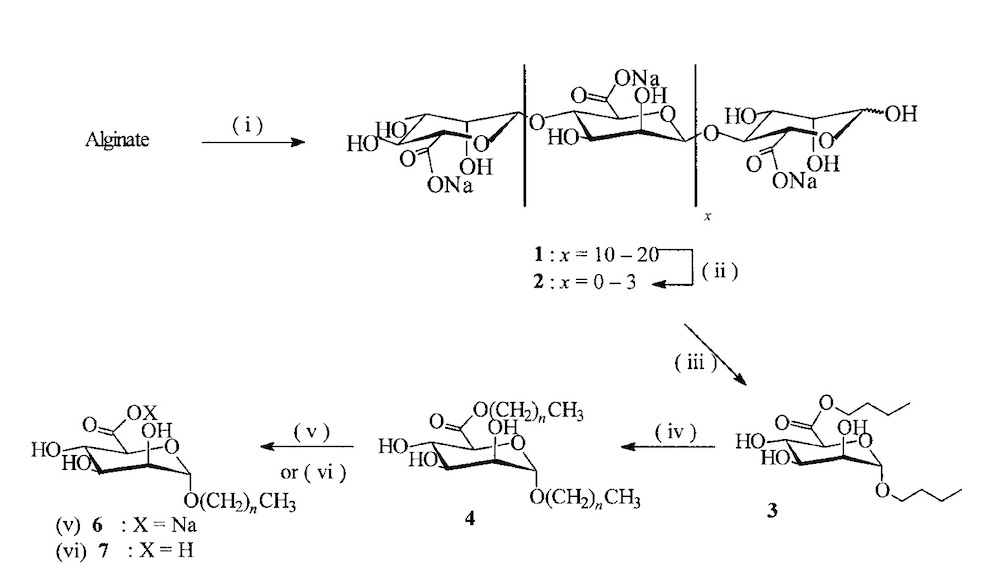
Rusell and other co-workers reported the biocompatible surfactants derived from alginate for different applications in detergents and cosmetics. Controlled acid hydrolysis of alginate led to saturated oligo‐mannuronates on a multi-gram scale. Following acid glycosidic bond hydrolysis, esterification, and stereo-controlled Fischer glycosylation in butanol with the related oligouronates provided, n‐butyl (n‐butyl α‐D‐mannopyranosiduronate). Double‐tailed amphiphiles were next gained from this crucial intermediate by transesterification/transglycosylation processes in fatty alcohols [13].
In this research, to separate the mannuronate units and obtain Oligo mannuronate with DP=5, THE solution containing mannuronate treated at pH = 3 in 100 °C for 8 hours then by acidification of the carboxyl group and esterification with butanol at a temperature of 112-115 °C the butyl ester intermediate called n-butyl-a-D-manno pyranosiduronate obtained.
To lengthen the chain and remove the butanol form and achieve a double-tailed amphiphilic compound, a fisher glycosylation reaction was required. In this study, this reaction performed in the presence of fatty alcohol, dodecanol, and MSA acid catalyst at 60 °C and under low pressure (2-5 mbar).
Treating, n-dodecyl-a-D-monno pyranosiduronate (4) in acidic or basic aqueous, led to a neutral and anionic single-tailed surfactant respectively with appropriate foaming properties and reducing of surface tension.
3-4-2- Synthesize of surfactant from kelp
For this purpose, 10-20 grams of fresh kelp leaves per 100 grams of water are required. The freshness of the leaves is very important because otherwise, chemical destructions of the surfactant may occur [4, 9].
In general, it is critical to avoid form factors that lead to the chemical degradation of the surfactant. These factors include different conditions like very high or very low pH (above 12.5 and below 4) and also temperatures above 60 °C.
Also to prevent osmotic degradation, the process must be done under a saline environment due to the saline growing environment of kelps.
Enzymes in this process include hemicellulase, cellulase, protease, and pectinase. Natural bacteria along with carbohydrates and sugar are also used to accelerate the process. Constant stirring in this process is required and so as to prevent any damages to kelp leaves, it is recommended to use compressed air flow instead of a mechanical method.
In order to detect the completion of the process, which usually takes 3-5 days, a refractometer is used. Microbial fermentation of kelp algae leads to the production of fatty acids that can be sulfonated or alkoxylated [4].
4- Properties of kelp surfactant
Kelp surfactant is an anionic surfactant that contains polysaccharides, betaine, natural oils, amino acids, and proteins.
The freezing and boiling points of kelp surfactant are between 0-6 and 104-98 °C respectively. Its best performance is at pH: 4-5.5 [9].
4-1- Surface activity
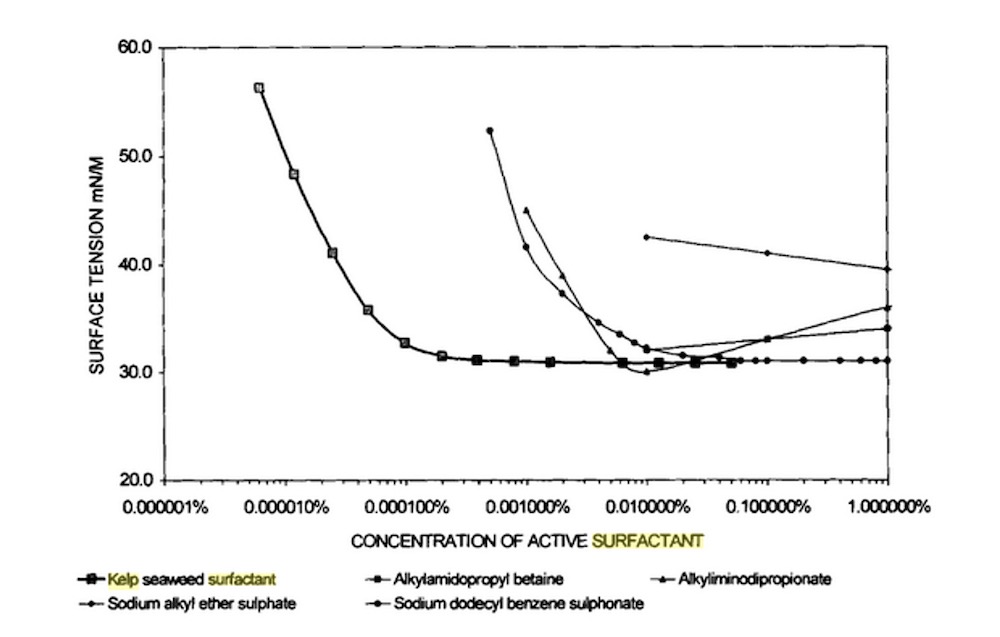
In order to investigate the surface activity of kelp surfactant, the reduction of surface tension in different concentrations of surfactant was investigated [9].

A comparison of the surface tension of kelp surfactant with sodium alkyl ether sulfate, sodium dodecylbenzene sulfonate, alkyl amidopropyl betaine, alkyl iminodipropionate is shown in figure 7.It is concluded that kelp surfactant has the ability to reduce the surface tension at a very low concentration in comparison to other surfactants.
5- Kelp surfactants applications
Kelp surfactant has proven to have a great ability to clean metal and plastic surfaces and also it is a suitable choice to remove grease from concrete without any damages to the surface. One of the main applications of kelp surfactant is in industrial detergents, grease, and oil cleaners. The advantages of this surfactant are:
1. Improving cleaning properties
2. Reducing the amount of surfactant used
3. Compatibility with the skin
4. Stability against strong acids and alkalis
5. Excellent solubilizing including body fat
The kelp surfactants has also been used in many shampoo formulations, skin cleaners bath and shower gels as it has not shown any toxicity and it has great compatibility with nonionic, anionic and amphoteric surfactants [14].
Kelp surfactants are also effective products in wastewater treatment, especially for removing fat and grease. Even a small quantity of kelp surfactant can emulsify fats [15].
6- Future of kelp surfactants
The development of surfactants based on natural renewable resources is a concept that has been gained attention, especially in green chemistry. Kelp surfactant has a high potential in various products and industries. The renewability and nontoxicity of this surfactant along with its special characteristics have made it a suitable alternative to chemical surfactants.
References
[1] Edwards, K. R., Lepo, J. E., & Lewis, M. A. (2003). Toxicity comparison of biosurfactants and synthetic surfactants used in oil spill remediation to two estuarine species. Marine Pollution Bulletin, 46(10), 1309-1316.
[2] Mattner, S. W., Wite, D., Riches, D. A., Porter, I. J., & Arioli, T. (2013). The effect of kelp extract on seedling establishment of broccoli on contrasting soil types in southern Victoria, Australia. Biological agriculture & horticulture, 29(4), 258-270.
[3] Leighton, D. L., Jones, L. G., & North, W. J. (1966, January). Ecological relationships between giant kelp and sea urchins in southern California. In Proceedings of the Fifth International Seaweed Symposium, Halifax, August 25–28, 1965 (pp. 141-153). Pergamon.
[4] D. Mundschenk,P.Reid , Kelp extraction biocatalyst and methodes , https://www.google.com/patents/US6284012 , 2001.
[5] Mulligan, C. N., Sharma, S. K., & Mudhoo, A. (Eds.). (2014). Biosurfactants: research trends and applications. CRC press.
[6] Hart, M. R., de Fremery, D., Lyon, C. K., & Kohler, G. O. (1978). Dewatering kelp for fuel, feed, and food uses: process description and material balances. Transactions of the ASAE, 21(1), 186-0190.
[7] Lee, K. Y., & Mooney, D. J. (2012). Alginate: properties and biomedical applications. Progress in polymer science, 37(1), 106-126.
[8] Paques, J. P., van der Linden, E., van Rijn, C. J., & Sagis, L. M. (2014). Preparation methods of alginate nanoparticles. Advances in colloid and interface science, 209, 163-171.
[9] Karsa, D. R., & Porter, M. R. (Eds.). (2012). Biodegradability of surfactants. Springer Science & Business Media.
[10] Soberón-Chávez, G., & Maier, R. M. (2011). Biosurfactants: a general overview. In Biosurfactants (pp. 1-11). Springer, Berlin, Heidelberg.
[11] Benvegnu, T., & Sassi, J. F. (2010). Oligomannuronates from seaweeds as renewable sources for the development of green surfactants. In Carbohydrates in Sustainable Development I (pp. 143-164). Springer, Berlin, Heidelberg.
[12] Matsubara, Y., Iwasaki, K. I., & Muramatsu, T. (1998). Action of poly (α-L-guluronate) lyase from Corynebacterium sp. ALY-1 strain on saturated oligoguluronates. Bioscience, biotechnology, and biochemistry, 62(6), 1055-1060.
[13] Roussel, M., Benvegnu, T., Lognoné, V., Le Deit, H., Soutrel, I., Laurent, I., & Plusquellec, D. (2005). Synthesis and Physico‐Chemical Properties of Novel Biocompatible AlkylD‐Mannopyranosiduronate Surfactants Derived from Alginate. European journal of organic chemistry, 2005(14), 3085-3094.
[14] Benvegnu, T., & Sassi, J. F. (2010). Oligomannuronates from seaweeds as renewable sources for the development of green surfactants. In Carbohydrates in Sustainable Development I (pp. 143-164). Springer, Berlin, Heidelberg.
[15] Singer, M. M., George, S., Jacobson, S., Lee, I., Weetman, L. L., Tjeerdema, R. S., & Sowby, M. L. (1995). Acute toxicity of the oil dispersant Corexit 9554 to marine organisms. Ecotoxicology and Environmental safety, 32(1), 81-86.
.