Md. Khalilur Rahman Khan / Email: [email protected]
1. Reality of Plastic Pollution
Textile, packaging, building and construction, consumer and institutional products, transportation, electrical and electronic equipment, and industrial machinery account for more than 350 million tons of plastics manufactured annually around the world. Despite the fact that plastics are important resources in many ways, their widespread use over the last several decades has resulted in a detrimental environmental impact due to low recycling rates after their initial use.
Despite this clear issue, the volume of plastic manufacture is predicted to expand steadily over the next few decades. Approximately 70% of worldwide plastics are currently found as waste. Only about 41% of post-consumer plastic material is recovered through recycling and incineration with energy generation, whereas 40% is disposed of in landfills and 19% ends up in the oceans or along coasts.
The long-term threat of toxic chemicals released into the environment by plastic pollution poses a major threat to ecological systems and health issues. Microplastics, which are made up of micron-sized particles degraded from waste plastics, have now become a major pollution in the ocean, posing a threat to hundreds of millions of marine life through ingestion, entanglement, and suffocation [1]. This vast accumulation of plastic waste has proven to be one of the most persistent environmental issues confronting modern society [2].

2. Biorecycling of Plastic
In face of current environmental concerns, recycling has become an essential component of the supply chain. The decomposition or degradation of organic substances by living entities such as microorganisms (bacteria, fungi, and marine microalgae) or enzymes is known as biodegradation. The primary recycling category is mainly concerned with the reuse of waste plastics, whilst the tertiary (recovery of industrially important elements such as monomers or additives) and quaternary (energy recovery) categories are concerned with the valorizing [1].
Surprisingly, the widespread release of plastics into the environment has resulted in the discovery of various biological systems capable of converting man-made polymers into carbon and energy sources. These bioscience implications for a circular materials economy can start with these plastic-degrading systems [3].
Biological recycling, which involves the enzymatic catalysis of plastic, has numerous advantages over traditional mechanical and chemical recycling procedures. Enzymatic degradation, for example, has a number of advantages, including benign process conditions, low energy input, and no need for hazardous chemicals or expensive apparatus, making it a very attractive alternative for PET recycling in the future [1].
3. PET Plastic
Polyethene terephthalate (PET), which is synthesized from petroleum-derived terephthalic acid (TPA) and ethylene glycol (EG), is the most common synthetic polymer manufactured today [3]. The molecule’s resilience, which made PET-based plastic a valuable multipurpose product, turned out to be a double-edged sword over time [2]. PET has been introduced into the environment in large amounts through its production and disposal, resulting in PET deposition in ecosystems all over the world [4].
4. Biorecycling of PET
PET can be biorecycled by degrading it with engineered microbes or enzymes under mild reaction conditions, then recycling the degraded monomers back into PET or upcycling them into more valuable compounds [1]. Cutinase and lipase family enzymes have generally been used in PET degradation research.
However, due to their poor affinities for PET, these enzymes were unable to degrade PET, resulting in limited PET degrading activity [5]. At mild temperatures, they display little activity, which is not encouraging for the industrial scale-up process [6]. Lipases, in particular, have the lowest hydrolysis activity of PET, owing to the fact that their catalytic centers are covered by lid structures, limiting the hydrolases’ contact and catalysis with the PET substrate.
On the other hand, cutinases have always had a high propensity to hydrolyze PET because of their vast substrate-binding pockets lacking lid structures, which allows PET to be combined with their active centers. Cutinases, however, degrade PET at high temperatures (50–70 °C) [7]. Moreover, PET is semicrystalline, with crystalline and amorphous structures coexisting. The biodegradation rate of plastics reduces with increasing crystallinity because enzymes can generally degrade the flexible amorphous domain [8].
5. Enzyme Cocktail
Enzyme cocktail is a mixture or combination of various enzymes that improves the effectiveness and efficiency of any catalytic reaction.
5.1 Enzyme Cocktail in Nature
In nature, synergistic enzyme cocktails that have developed over millions of years deconstruct recalcitrant polymers like cellulose and chitin. Interfacial biocatalysis is used to release soluble dimeric or oligomeric intermediates, and additional enzymes are used to convert the soluble intermediates to monomers for microbial uptake. Enzymatic deconstruction of resistant natural polymers, such as cellulose, hemicellulose, and chitin, occurs naturally through the action of synergistic enzymes released by bacteria.
These combinations usually comprise a subset of enzymes that act directly on solid polymeric substrates via interfacial enzyme mechanisms, and complementary enzymes (e.g., -glucosidases) that further transform solubilized intermediates to monomeric constituents, as seen in fungal cellulase systems for cellulose depolymerization (e.g., cellobiose hydrolysis to glucose). Natural microbial systems have developed over millions of years to breakdown recalcitrant polymers optimally [3].
5.2 PET Eating Enzyme Cocktail
5.2.1 Ideonella sakaiensis
Yoshida et al. identified and isolated the bacterium Ideonella sakaiensis 201-F6 from a subculture of the microbial consortium No. 46 in 2016. This bacterium is Gram-negative, aerobic, non-spore-forming, rod-shaped, and its hydrolytic activity was examined on a low-crystallinity PET film (Tg: 77°C, Tm: 255°C, crystallinity: 1.9 %) after 48 hours of incubation at 30°C.
Two enzymes, Polyethylene terephthalate hydrolase (PET hydrolase or PETase) and mono (2-hydroxyethyl) terephthalic acid hydrolase (MHETase), working in a synergistic way, were found to be involved for the degradative function [6]. These two enzymes are part of a unique enzyme system observed in Ideonella sakaiensis, and their combined efforts allowed the bacterium to thrive while using PET as its primary carbon source, proving that there is hope for a viable solution to the problem of plastic waste degradation and disposal [2,9].
When MHETase is included in the procedure, the enzyme synergy results for the I. sakaiensis (PETase/MHETase) system show a clear improved performance [3].
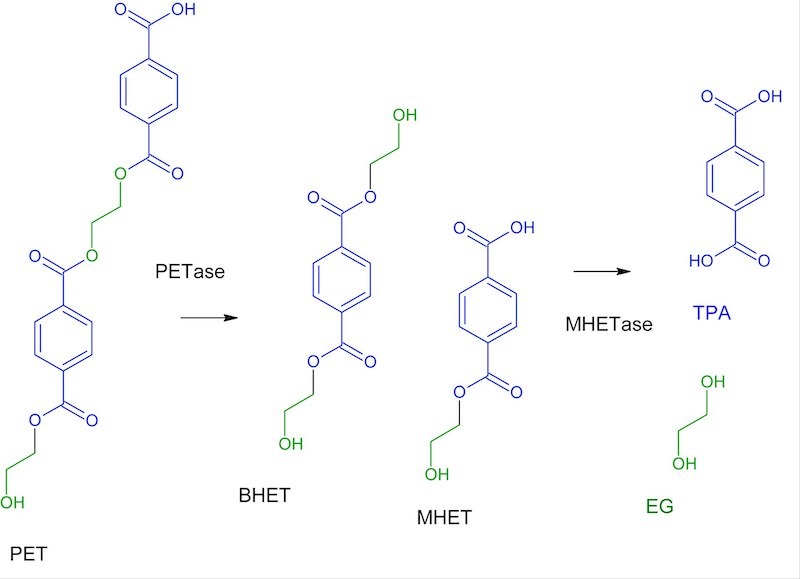
5.2.2 PETase & MHETase
PETase in I. skaiensis belongs to the cutinase family. Despite the fact that the PETase’s crystal structure is quite similar to that of other phylogenetically related cutinases, the PETase’s catalytic efficiency is many times higher than that of the other recognized cutinases.
MHETase The PETase-mediated breakdown of PET produces mono (2-hydroxyethyl) terephthalic acid, or MHET. MHETase contains several structural motifs with its closest fungal homologs—the tannase/ esterase family of enzymes [2].
5.2.3 PET degradation
Microorganisms adhere to the surface of PET films during biodegradation, then secrete extracellular PET hydrolases, which connect to the films and commence the biodegradation process. PET hydrolases (e.g., PETase) work on the ester bond of PET, hydrolyzing it into terephthalic acid (TPA) and ethylene glycol (EG) and yielding incomplete hydrolysis products such mono(2-hydroxyethyl) terephthalate (MHET) and Bis-(2-hydroxyethyl) terephthalate (BHT) (BHET). Under the action of MHETase, BHET can be further degraded into MHET (from BHET), and MHET can be further broken into TPA and ethylene glycol [7,2].
Weight loss, CO2 generation by PET catabolism, surface morphological changes in films identified by scanning electron microscopy (SEM), and a growth in the number of surface functional groups determined by X-ray photoelectron spectroscopy (XPS) are all indicators of PET film degradation [8].
5.2.4 Enzyme Cocktail Modification
The addition of aromatic residues like Phe can boost PETase’s substrate affinity, whereas the addition of charged amino acids like His and Glu can strengthen the protein’s thermal stability. When compared to its wild type counterpart, a triple mutated Ideonella PETase (IsPETase) has a melting temperature increase of more than 8° C, greater thermal stability, and 14-fold stronger PET degradation ability. In MHETase, however, replacing aromatic amino acids like Phe with aliphatic amino acids like Val or Ilu allowed the enzyme to accept BHET as a substrate, improving the enzyme’s versatility.
As a result, an enzyme cocktail containing an engineered PETase as well as engineered and wild-type MHETase can be used as a bioremediation agent to degrade PET wastes [2]. PETase and MHETase were found to aid in the high-efficiency biodegradation of PET at ambient temperatures. The structures of these two enzymes have been extensively studied at this time [7].
5.2.5 Potentiality
The discovery of I. sakaiensis opens up the possibility of a low-energy bioremediation solution for plastic waste [1]. Surface treatment of PET fibers for texturizing and staining textiles is a possibly alternative application [8].
5.2.6 Challenges
PETase has been found to have the highest PET degradation activity under mild circumstances of all PET degrading enzymes discovered to date, however its limited thermal stability restricts the enzymatic degradation of PET in practice. Enzymes should be modified to become kinetically faster and thermodynamically more stable in order to digest PET on a big scale [6].
PET wastes from various applications may contain significantly different contaminants and physical attributes, such as shape, crystallinity, glass transition temperature, and mechanical strength, resulting in significantly different biodegradation efficiencies under the same operation and control system. Further research into different PET sources is expected. In fact, the absence of sufficient knowledge and experience in scaling up present biodegradation technology is the third main issue [1].
Standard commercial methods such as enzyme immobilization, enzyme entrapment, or encapsulation also complicate the interaction between PETase and PET. The prospects for a circular bio-based economy of PET are projected to pave the way for a sustainable plastics sector in the future.
References:
1. Soong, Ya-Hue Valerie et al. “Recent Advances in Biological Recycling of Polyethylene Terephthalate (PET) Plastic Wastes.” Bioengineering (Basel, Switzerland) vol. 9,3 98. 27 Feb. 2022, doi:10.3390/bioengineering9030098
2. Maity W, Maity S, Bera S, Roy A. Emerging Roles of PETase and MHETase in the Biodegradation of Plastic Wastes. Appl Biochem Biotechnol. 2021;193(8):2699-2716. doi:10.1007/s12010-021-03562-4
3. Knott, Brandon C et al. “Characterization and engineering of a two-enzyme system for plastics depolymerization.” Proceedings of the National Academy of Sciences of the United States of America vol. 117,41 (2020): 25476-25485. doi:10.1073/pnas.2006753117
4. Yoshida, Shosuke et al. “A bacterium that degrades and assimilates poly(ethylene terephthalate).” Science (New York, N.Y.) vol. 351,6278 (2016): 1196-9. doi:10.1126/science.aad6359
5. Joo S, Cho IJ, Seo H, et al. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat Commun. 2018;9(1):382. Published 2018 Jan 26. doi:10.1038/s41467-018-02881-1
6. Berselli A, Ramos MJ, Menziani MC. Novel Pet-Degrading Enzymes: Structure-Function from a Computational Perspective. Chembiochem. 2021;22(12):2032-2050. doi:10.1002/cbic.202000841
7. Qi X, Yan W, Cao Z, Ding M, Yuan Y. Current Advances in the Biodegradation and Bioconversion of Polyethylene Terephthalate. Microorganisms. 2022; 10(1):39. https://doi.org/10.3390/microorganisms10010039
8. Yoshida S, Hiraga K, Taniguchi I, Oda K. Ideonella sakaiensis, PETase, and MHETase: From identification of microbial PET degradation to enzyme characterization. Methods Enzymol. 2021;648:187-205. doi:10.1016/bs.mie.2020.12.007
9. Bethany Halford, Bacterium’s enzymes break popular plastic’s bonds to form recyclable monomers, C&EN, 2016, 94 (49), p 25December 19, 2016. Available from https://pubs.acs.org/doi/pdf/10.1021/cen-09449-cover3
10. Palm, Gottfried & Reisky, Lukas & Böttcher, Dominique & Müller, Henrik & Michels, Emil & Walczak, Miriam & Berndt, Leona & Weiss, Manfred & Bornscheuer, Uwe & Weber, Gert. (2019). Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nature Communications. 10. 10.1038/s41467-019-09326-3.





















